First-Ever Saliva Swab Test Aims to Help Speed Up Autism Diagnostic Process (Here’s How It Works)
July 7, 2020
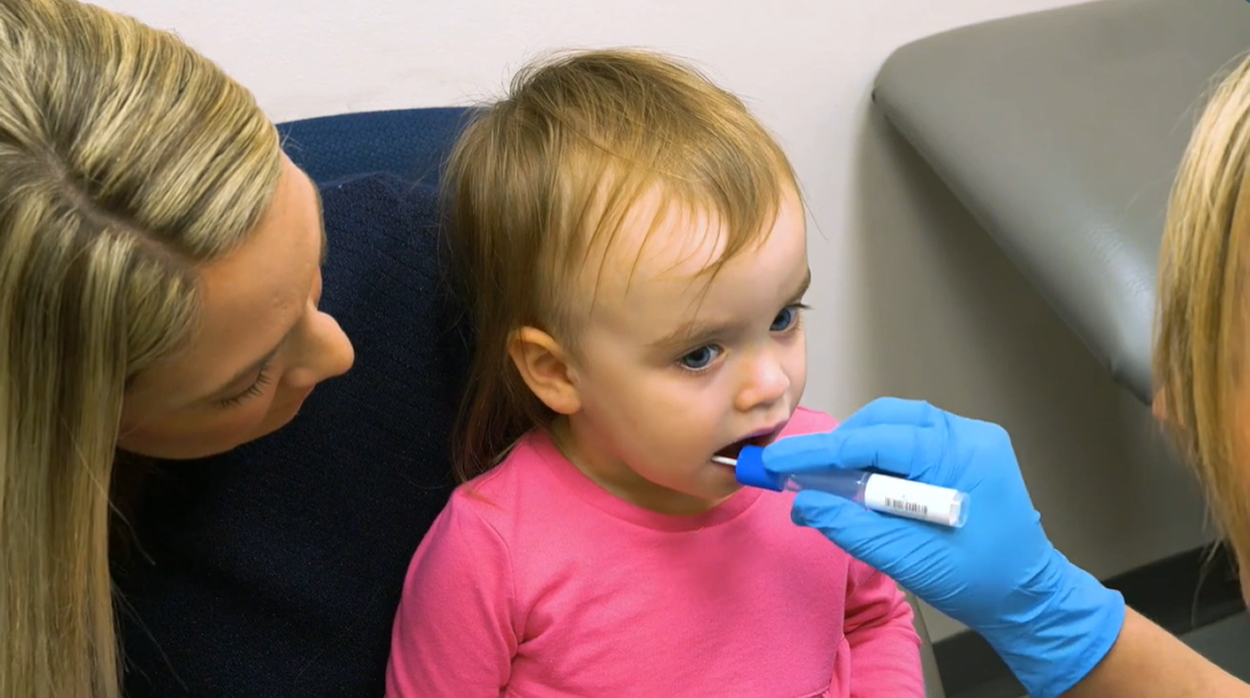
Sounds a little hard to believe, doesn’t it? Don’t worry, we thought so too. Imagine a simple test that accurately delivers health care providers a probability of autism diagnosis in a matter of weeks.
Well it’s called Clarifi ASD™, and since the beginning of 2020, primary care physicians across the country have been offering the test to children between the ages of 18 months through six years (72 months) old, where the children have a positive screening test or underlying concern for autism.
The Need
The average age of diagnosis in the U.S. is typically between four and five years old. Studies have shown that many children who receive a diagnosis and intervention at an earlier age have better outcomes. Clarifi ASD aims to help identify children with autism within the second year of life so that they can get earlier access to the services and benefits they need to maximize their potential.
So how does this work and why are we just hearing about it now?
A team of researchers at SUNY Upstate Medical University and Penn State College of Medicine discovered that the composition of certain RNA molecules in the saliva was different in children with autism, compared to their peers.
Using next-generation sequencing, the researchers analyzed saliva samples for specific RNA molecules, as well as bacteria from the microbiome, to accurately determine the probability of a child having autism.
An Epigenetic Test
Clarifi ASD is not a genetic test. Rather, it is the product of scientific advancements in the area of epigenetics. While your genetic code, your DNA, does not change, the way those genes are expressed (turned “on” or “off”) can be altered by internal biological processes and environmental influences.
This is called epigenetics, which involves factors that influence how genes are turned on and off. Specifically, Clarifi ASD measures the activity of genes by measuring specific RNAs and bacteria in the saliva to provide a probability of an autism diagnosis. Both internal biological processes and environmental influences— like food, chemicals and exercise —can affect gene expression.
The peer-reviewed research discovered that certain RNA molecules that regulate gene expression and reflect interactions between a patient and his or her environment, were different in children with autism.
Before being released to physicians for public use, the Clarifi ASD test and its associated algorithm were put through a series of rigorous studies to make sure it really worked. The researchers published several peer-reviewed papers culminating in the 2018 paper titled, “Validation of a Salivary RNA Test for Childhood Autism Spectrum Disorder,” which was published in the journal Frontiers in Genetics. This new autism test was then developed at an independent, CLIA certified laboratory as a laboratory-developed test and was released to health care providers across the United States to assist in the diagnostic process.
The Research
Clarifi ASD is supported by peer reviewed research. Read the published papers below.
Currently, thousands of parents each year flock to pediatricians for referrals to specialists and wait for months (and sometimes years) to get an official autism diagnosis. The Clarifi ASD test is designed to identify children with a high likelihood of having autism, as part of a well-child visit, in order to begin effective interventions much earlier than 4 years of age.
Research shows that these support services and therapies can make a powerful impact on long-term outcomes for children with autism, especially when applied before the age of three. The sooner a child receives autism support services such as behavioral interventions, occupational therapy, and speech therapy, the better chances that child has to thrive – even into adulthood.
“Interventions initiated before three years of age may have a greater positive impact than those begun after the age of five years,” a study published in the American Academy of Pediatrics reported.

Why is this?
According to the National Institutes of Health (NIH), a young child’s brain is still forming at or before preschool age, making it more “plastic” than at older ages. Because of this, intervention services have a better chance of being effective in the long term, when applied before the age of three.
According to the NIH, “The sooner a child gets help, the greater the chance for learning and progress. In fact, recent guidelines suggest starting an integrated developmental and behavioral intervention as soon as ASD is diagnosed or seriously suspected.”
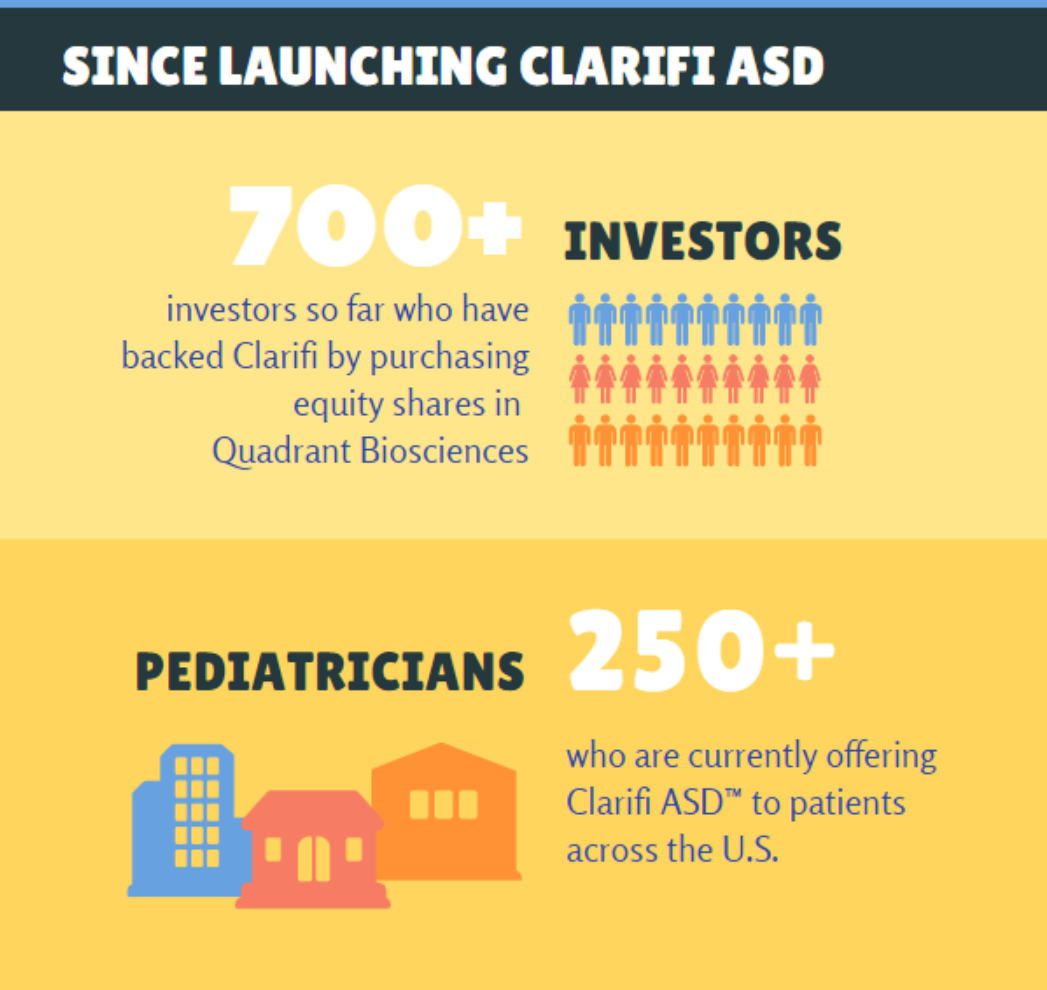
Quadrant Biosciences, the company behind the Clarifi autism saliva test, is working toward getting the test covered by major insurance carriers including Medicaid in order to make the test available to all patients who need it. While it is not yet covered by insurance, it is being offered at an introductory price of $989.
Quadrant Biosciences is currently offering investment shares to the public at $3 per share in order to fund its ability to continue to grow and expand the development and regulatory approval of this and other epigenetic diagnostic tests.
A few more interesting facts…
- Clarifi ASD is the world’s first autism saliva test
- Clarifi ASD is backed by peer-reviewed research & CLIA certified
- Quadrant is now developing tests based on the same epigenetic model for diagnosis of Parkinson’s disease, concussion, and anorexia nervosa.
- Quadrant has filed more than 29 patent applications regarding this work
Learn more about investing in Quadrant Biosciences and read important disclosures about the risks associated with this investment in the offering circular found here:
Securities offered through North Capital Private Securities, member FINRA/SIPC





